Products
Formulation
Sodium Lauryl Sulfate (SLS)30% PADAPON Sl-30
what is “SLS” (Sodium Lauryl Sulfate ) :
Sodium Lauryl Sulfate:
In this article, we’ll discuss Sodium Lauryl Sulfate (SLS) as a widely-known anionic surfactant. SLS is usually recognized for its exceptional foaming, emulsification, and cleaning capabilities. Looking into its chemical structure, SLS is characterized by a long alkyl chain, a sulfate group, and sodium. This chemical composition provides a variety of applications from cleaning and self-care products to agriculture, construction, textiles, and food processing. Safety concerns about SLS, especially in prolonged skin contact, are addressed. We’ll emphasize the need for adherence to recommended concentrations and usage guidelines
more info about SLS:👇
| 🔵 Appearance: | colorless or light yellow |
| 🔴 Molecular weight : | 302 (g/ mol) |
| 🔵 Active substance(%) : | MIN 28 |
| 🔴pH (10%) : | 6-9 |
Product specifications
| PH | 6-9 |
|---|
Introduction and general information
Introduction of Sodium Lauryl Sulfate :
Sodium Lauryl Sulfate: stands as one of the most extensively utilized anionic surfactants. It’s a sodium salt categorized within the organosulfate family. Similar to other anionic surfactants, it has a long alkyl chain (typically C11-13), which functions as a hydrophobic or lipophilic segment, alongside a sulfate group that takes part in hydrophilic interactions.
A considerable portion of Sodium Lauryl Ether Sulfate’s reputation can be attributed to its remarkable foaming capabilities, exceptional emulsification qualities, and strong cleaning efficacy. This remarkable surfactant activity makes SLS highly versatile, suitable for a wide range of tasks, and particularly effective in removing stubborn stains.
Appearance of sls:
SLS comes in the form of a colorless to pale yellow liquid. The concentration of SLS in the solution has a strong influence on its viscosity. SLS can also be synthesized in the form of a solid powder.
Chemical structure and mechanism of action:
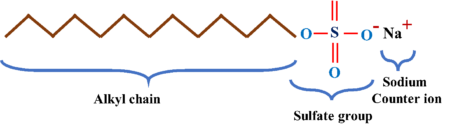
Sodium lauryl sulfate has the chemical formula CH3-(CH2)n-OSO3-Na, where ‘n’ is typically set to be between 11 and 13. The chemical structure of the product can be divided into three parts:
- Long alkyl chain: A long lauryl or alkyl chain increases the product’s hydrophobicity. They have the potential to react with fat, dust, and other nonpolar compounds. A shorter alkyl chain would result in more water solubility (higher HLB), whereas a longer alkyl chain would prepare the product for high-temperature applications. In order to attain optimal qualities, commercial SLS products usually set the number of carbons (in the alkyl chain) to 11-13.
- Sulfate group: The sulfate component of the molecule enhances the product’s hydrophilic traits. This hydrophilic head enables the molecule’s dissolution in water and other polar solvents. The anionic characteristics of SLS originates from its sulfate group.
- Sodium: Na is the counter ion used in the synthesis of SLS. It neutralizes the product’s electrical charge and converts it to an amphiphilic structure. Sodium, as a counter ion, has a high affinity for water, which increases the product’s solubility.
The final qualities are affected by concentration and application temperature. When the concentration of SLS solution is increased to 40%, hexagonal phase development occurs, resulting in greater viscosity. The lamellar structure of SLS would marginally decrease viscosity at concentrations exceeding 70%.
Its critical micelle concentration of roughly 8 mM in water makes it a good candidate for usage in a variety of industries.
Chemical structure of Sodium Lauryl Sulfate:
Applications and target market of Sodium Lauryl Sulfate:
1- Cleaning and Self-care
Because of its excellent emulsification, foaming, and cleansing qualities, SLS is an excellent choice for self-care products, detergents, shampoos, soaps, and bath gels. As previously indicated, the comparatively low CMC value of SLS enables producers to employ it as a cost-effective ingredient. Another reason that SLS is an appropriate solution in this scenario is the high value of the micelle ionization fraction.
2- Polymer, Paint, Resin, Coatings
Latex formulations and emulsion coatings commonly incorporate a surfactant, with SLS standing as a prevalent inclusion in these setups. SLS is a key ingredient in several water-based paints. Another use for SLS may be emulsion polymerization. SLS, on the other hand, is used in some polymeric systems to provide component stability and dispersion.
3- Agriculture
Some insecticides and herbicides contain sodium lauryl sulfate in their composition. It might be used as a repellent for fleas and ticks or as an adjuvant to aid other chemicals. SLS might also be employed to assure colloidal solution stability. Moreover, it has the potential to be employed as a foaming agent in sprays and aerial applications.
4- Construction
SLS might be utilized as a foaming agent in foam concretes. SLS is capable of producing stable foams with a wide variety of densities. Soil remediation is another newly formed area of interest. It is necessary to manage soil polluted by petroleum products. This pollution might occur as a result of a leak, spills, or automobile accident. SLS, in conjunction with other surfactants or nanoparticles, might be utilized to remove contaminants and heal the soil.
5- Textile
SLS might be used as a surfactant or salt in the textile dyeing process. On the other hand, as a wetting agent, it enhances the wettability of the materials, resulting in improved dye absorption. As previously stated, SLS might be employed as a dispersion agent to increase component stability.
6- Food processing
SLS is used directly in the manufacturing process of several food items such as marshmallows and dry egg derivatives. In fact, it might be employed as a foaming agent or as an excipient to aid the process by ensuring the components’ steady dispersion.
Safety concerns of sls:
One of the most frequently expressed concerns regarding SLS is its safety. In fact, SLS is FDA authorized, Cosmetic Ingredient Review (CIR) deemed it safe in concentrations up to 10%, and ISO created criteria for SLS safety usage. However, there have been several instances of skin irritation following the use of SLS-containing cosmetic and self-care products. As a result, producers must adhere to strict regulations in order to utilize the permissible concentration of SLS in their goods. It should be mentioned that the time of direct contact is another factor that influences SLS safety. SLS might be used in items that do not come into touch with the skin for an extended period of time.
Specialized information request form
Need more information about this product?
Login to account
Log in to the user account to view additional technical data sheet, safety information, analysis sheet.
Request more information
Certificates

NACI

ISO 45001

ISO 14001

ISO 9001

isiri