Products
Formulation
Sodium Lauryl Ether Sulfate(2EO) 70%(SLES) PADAPON SE-270
what is “SLES” ( Sodium Lauryl Ether Sulfate 70% ) :
In this article, we explore Sodium Lauryl Ether Sulphate (SLES), a versatile surfactant widely used in industries ranging from construction to cosmetics.
We’ll examine its chemical structure, diverse applications, and unique properties like foaming and emulsifying. Safety concerns surrounding ” SLES “ and its distinction from “SLS“ are also addressed, highlighting its approved short-term safety and considerations for long-term use.
more info about SLES 70 2EO:
| 🔵 Appearance: | White/light yellow paste |
| 🔴 Molecular weight : | 384 (g/ mol) |
| 🔵 Active substance : | 68-72 |
| 🔴C12 (wt.%) : | 65-75 |
| 🔵 C14 (wt.%) : | 20-30 |
| 🔴pH : | 7-9 |
| 🔵 Dioxane (ppm) : | Max50 |
Product specifications
| Molecular weight | 384 (g/mol) |
|---|---|
| dioxane (ppm) | Max 50.0 |
Introduction and general information
Introduction of SLES 70% 2eo
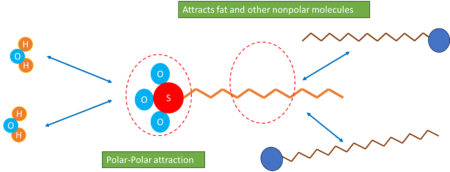
Sodium Lauryl Ether Sulphate, also known as Sodium Laureth Sulphate or ” SLES “, is one of the most common and versatile ” anionic surfactants “ widely used across various industries.
As a member of the Alkyl ethoxy sulfates (AES) family, it shares similarities with most surfactants, comprising a hydrophilic part and a hydrophobic tail. The hydrophilic structure allows SLES to dissolve in water and other polar solvents, while the hydrophobic part interacts with nonpolar materials.
Features and Advantages of SLES 70% :
- Removing fat as a foaming, cleansing and emulsifying agent
- Solubility in water in various proportions
- Compatibility with a variety of surfactants
Appearance :
SLES can be produced in various forms, such as viscous liquids, pastes, powders, or even as a fine powder, appearing colorless or pale yellow, cream, or white.
Chemical Characteristics
The chemical structure of SLES, presented below, has a general molecular formula of CH3(CH2)10CH2(OCH2CH2)nOSO3Na, where ‘n’ represents the length of the ethoxy group, typically designed to be 1, 2, or 3. Altering ‘n’ can lead to changes in the chemical and physical properties of SLES. The presence of a long hydrocarbon chain with 11 carbons and ethoxy groups, along with the sulfonate group acting as an anionic hydrophile agent, contributes to the unique properties of SLES. By adjusting the amount of ethoxylation and ethoxy value, different physical and chemical characteristics can be achieved.
Structure and Mechanism of Action
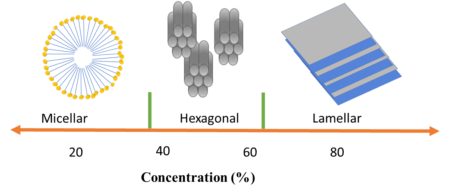
As evident from the chemical structure, SLES consists of a long hydrocarbon chain comprising 11 carbons with ethoxylate groups. The sulfonate group functions as an anionic hydrophile agent. Changing the ethoxylation and ethoxy values can lead to diverse physical and chemical properties in SLES. Additionally, SLES can be produced in various concentrations. The concentration of the product influences its physical qualities, such as viscosity and morphology. Concentrations below 20% exhibit Newtonian behavior, while higher viscosities demonstrate shear-thinning behavior. Moreover, increasing the concentration could result in the formation of hexagonal and lamellar structures in the solution.
SLES MECHANISM OF ACTION
As a cleaning agent, the long alkyl chain of SLES, being non-polar in nature, effectively interacts with nonpolar molecules such as various types of dirt and pollutants. This interaction lowers the surface tension and consequently, reduces the adhesion of pollutants to the substrate. Conversely, the polar head of SLES interacts with water and other polar solvents, enabling them to dissolve and remove pollutants.
Sodium Lauryl Ether Sulfate phase in different concentrations
Another valuable function of SLES is the formation of micelles through the aggregation of alkyl chains. Because of its micellar structure, SLES may be employed as a foaming agent and detergent in a variety of applications.
The foaming behaviour of SLES remains unaffected even in hard water. In fact, SLES could function in wide-range of environmental tolerance. This fact contributes to its popularity.
The outstanding performance of SLES in micelle formation also allows it to serve as an emulsifier As a result, Sodium Lauryl Ether Sulfate is well acknowledged for its efficiency as an essential component of emulsions.
By reducing ethoxylation and ethoxy groups, the size of the micelles formed by SLES is decreased. Additionally, lower amounts of ethoxy groups reduce the number of aggregated molecules required to form a spherical micelle.
Another feature of SLES is its capacity to remain stable even at low pH levels. SLES has the ability to maintain stability even at low pH values. Manufacturers may safely use SLES into their products thanks to these characteristics.
As mentioned earlier, the emulsifying, surface treatment, foaming, and detergent properties of SLES make it a versatile and suitable choice for various industries.
Application and target industry of SLES 70%:
1– Polymer, Plastics, Resin, Paint, and Coating:
SLES finds application as an emulsifier in emulsion polymerization systems and can also be employed in emulsion co-polymerization systems. It might also be employed as a surfactant in waterborne resins and polymeric colloidal systems. Additionally, SLES could be utilized as a wetting agent in the coating industry.
2– Construction
Because of their lightweight and excellent mechanical properties, foam concretes are gaining popularity in the building sector. In this regard, SLES is an ideal foaming agent for such systems. Furthermore, in soil bearing applications, Sodium Lauryl Ether Sulfate can be applied to create foam soil. So Sodium Lauryl Ether Sulfateis a useful product in the mechanized tunneling industry.
3– Self-Care and Cleaning
Sodium Lauryl Ether Sulfate is predominantly used in cleaning industries and self-care products. it is the key ingredient in many commercial products such as industrial cleaners and household detergents (shampoos, soaps, dishwashing liquids and …). Due to its chemical composition, SLS has a strong cleaning ability and is a suitable solution for the self-care and cleaning products.
Notably, one of the key advantages of Sodium Laureth Sulfate is its ability to act as a rheology modifier, as its high viscosity allows manufacturers to adjust and modify their product’s rheology using different concentrations of SLES.
4– Cosmetics
In the domain of emulsions, Sodium Lauryl Ether Sulfate70% is a commonly used ingredient, serving as an effective stabilizer. The ability of Sodium Lauryl Ether Sulfate to guarantee long-term stabilization of emulsions makes it a proper choice for this purpose.
5– Petroleum
petroleum contamination in soil is a recently raised challenge. Employing SLES as a functioning surfactant is a solution to address the problem through foaming and cleaning processes. Surfactants are usually used alongside nanoparticles and other components.
Furthermore, one of the primary benefits of using sodium lauryl ether sulfate is its rapid soil remediation cycle. Remediation cycles referred to the time or cycles of curing treatment.
Moreover, the issue pressure drop (drag) during transportation is a major concern in the petroleum industry. introducing surfactants to the flow, reduces the turbulence ignorantly. The lower turbulence results in reduced drag and cost savings. SLES is an ideal surfactant in this regard.
6– Fire Protection
In fire protection tools, most firefighting capsules contain foaming agents and surfactants. SLES as a powerful foaming agent finds application in these tools. SLES could enhance the foaming and fire protection of such systems.
Safety concerns of Sodium Lauryl Ether Sulfate 70%
The AES family, particularly SLS (Sodium Lauryl Sulfate), has faced suspicions of being harmful or carcinogenic. However, obtained results of various research confirm that the ethoxylation process of SLS to form SLES make it less irritating and milder. Methodological research and meta-analyses further approve the safety of using SLS within specific concentrations.
Despite the approved safety of the SLES in short-time research, its long-time safety is somehow controversial. Regardless of the presented arguments, the primary cause of concern is 1,4-Dioxane, a byproduct of the manufacturing process. However, proper formulation and production regulation can effectively eliminate any associated risks.
Specialized information request form
Need more information about this product?
Login to account
Log in to the user account to view additional technical data sheet, safety information, analysis sheet.
Request more information
Certificates

NACI

ISO 45001

ISO 14001

ISO 9001

isiri